De- mixing the Mixifusor with Tivatrainer

Tivatrainer has been designed not only for educational purposes but also to explore alternative methods of administering Intravenous anesthesia. A key focus in many IV anesthesia workshops has been the practice of combining drugs in a single syringe, such as propofol/ketamine (ketofol), propofol/alfentanil, and more recently, propofol/remifentanil combinations suggested specifically for pediatric anesthesia. This innovative approach should offer several benefits, including the need for only one pump and infusion line, avoiding issues with dead space in the infusion line, and reducing the number of reflux valves required. It is suggested that this method may also result in less pain upon propofol injection due to the faster onset of remifentanil compared to propofol, although there may be some uncertainty regarding this claim as pain upon injection is typically a localised phenomenon, while the pain-reducing effect of remifentanil is more centrally focused.
However, the drawbacks of utilising this Mixifusor system are comprehensively discussed in an article by Tony Absalom and colleagues.
These disadvantages include challenges related to physical drug mixing, an increased risk of drug preparation errors, potential legal implications, and most importantly, the potential for unexpected, uncontrolled, and undesired changes in drug concentrations in the added drug resulting from pharmacokinetic differences between the control drug (TCI propofol) and the dependent mixed drug (remifentanil).
In the Absalom publication, the paedfusor kinetics are utilised for the administration of TCI propofol, while the adjunct medication, remifentanil at a concentration of 0.005 mg/ml, is modeled using the Eleveld pharmacokinetic model for remifentanil.
The aim is to showcase the user-friendly nature of Tivatrainer in simulating combinations of intravenous medications. In this context, I will replicate the scenario from the publication, replacing the paedfusor model with the Eleveld pharmacokinetic model for propofol. A detailed walkthrough of the simulation process will be provided in the next blog.
However, before proceeding, it is crucial to verify that the simulations conducted with Tivatrainer align with those presented in the publication. Here lies the challenge: the simulations in the publication cannot be replicated using Tivatrainer, necessitating a thorough examination of the publication.
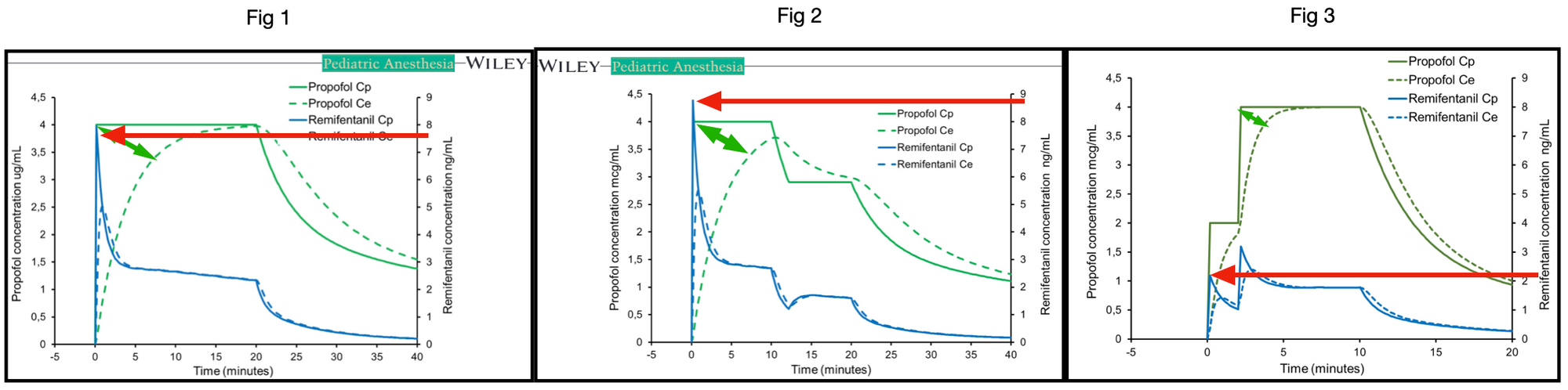
Upon analysing figure 1 in the publication, the notable equilibration time between blood and effect is strikingly prolonged. In theory, at one to five times the Thalfkeo, the effect site concentration should approximate 50%, 75%, 87.5%, 93.75%, and 96.875% of the blood concentration when utilising the semi-steady state established by Target Controlled Infusion. Assuming the Thalfkeo is based on the publication by Munoz et al., akin to commercially available TCI devices, the keo is estimated at 0.91 min-1, corresponding to a Thalfkeo of 0.76 minutes
Therefore, after 5 times 0.76 minutes, equilibration should occur with 96.875% of the blood concentration within 3.8 minutes. However, this is evidently not the case in figures 1 and 2. Equilibration occurs somewhere between 15 and 20 minutes, suggesting a Thalfkeo of approximately 3 minutes. Indeed in the text it is stated that the keo used is the keo from the adult Marsh model being 0.26 /min that corresponds with a Thalfkeo of 2.67 min. It must be noted that this keo is estimated for adults using auditory evoked responses and that both the aforementioned study of Muñoz and the following study
Jeleazcov, C. et al. Pharmacodynamic modelling of the bispectral index response to propofol-based anaesthesia during general surgery in children. Br J Anaesth 100, 509–516 (2008) found a faster equilibrium between effect and blood for the peadfusor model.
In contrast the observed Thalfkeo in figure 3 is notably shorter, surpassing even the Munoz thalfkeo of 0.76 minutes. This discrepancy is visually apparent.
The three-compartment models employed assume linear kinetics, where doubling the dose results in a proportional increase in concentration. In figure 1, the peak blood concentration of remifentanil reaches 7.6 ng/ml when propofol is targeted at 4 µg/ml. Conversely, in figure 2, with the same propofol target of 4 µg/ml, the estimated peak remifentanil concentration rises to 8.7 ng/ml. In figure 3, halving the target propofol from 4 to 2 µg/ml, the expected peak remifentanil concentration should also half from 7.6 to around 3.8 ng/ml. While the Tivatrainer simulation predicts indeed 3.9 ng/ml, the graph in figure 3 displays a peak closer to 2.2 ng/ml. Consequently, less propofol is administered to achieve the propofol concentration via Target Controlled Infusion (TCI) when calculated using the paedfusor model. Notably, upon re-simulation with Tivatrainer, differing blood concentrations for propofol are predicted when the target is adjusted to 0, as clearance and redistribution processes influence the decay of propofol according to the utilised pharmacokinetic model. It is evident that the simulation in figure 3 does not align with the kinetics of the paedfusor model. In videos 1a 1b and 2a and 2b, Tivatrainer simulations are superimposed on the publication graphs, revealing identical propofol blood concentrations but discrepancies in the propofol effect site between Tivatrainer and the published data in fig 1.
Video 1a: Re-simulation of propofol in fig 1: identical blood concentrations but faster effect site (solid green line) than published (dotted green line)
As propofol TCI is blood-controlled, the effect site does not impact the co-administered amount of remifentanil. The Tivatrainer simulation of remifentanil mirrors the administration depicted in figure 1.
video 1b: Re-simulation of remifentanil in the fig 1 of the publication. Tivatrainer simulation of blood(solid pink line) and effect(dashed pink line) match the remifentanil concentrations in the publication.
However, in figure 3, the Tivatrainer simulation diverges in all aspects, suggesting the utilisation of alternative kinetics not the peadfusor. The blood and effect site concentration in fig 3 do not align with the blood and effect site concentration in the decay phase of the TCI propofol.
vide2a: Resimulation of the TCI propofol in fig 3 of the publication. The blood equilibration is faster than in the peadfusor model and also the decay after switching the target to zero is faster.
video 2b: re-simulation of the remifentanil concentration in fig 3. Although the initial target is half of the target in fig 1 and 2 the resulting initial peak remifentanil in the publication is less than half of the initial peak remifentanil concentration in fig 1 or 2 of the publication( 2.1 ng/ml). Theinitial peak remifentanil in the tivatrainer re-simulation(pink lines) is correctly about half the peak remifentanil concentration in fig 1 or fig 2
Even without re-simulation, a simple visual inspection exposes the inconsistencies among figures 1, 2, and 3. While I concur with the conclusions of the publication, the simulations utilised lack the reliability necessary to draw definitive conclusions regarding the substitution of the Eleveld model for the paedfusor model.
It is worth mentioning that Tivatrainer proved to be a highly valuable tool in examining the irregularities present in the aforementioned publication. Should there be a need to validate a drug simulation graph, Tivatrainer can be utilised for this purpose. During the review of publications, discrepancies were identified on multiple occasions between the dosage utilised in the study under review and the purported concentrations in blood or effects.
In this particular instance, I employed the export feature of Tivatrainer to create CSV files that can be imported into all contemporary spreadsheet applications. By performing some basic manipulations, a graph was generated with axis settings akin to those in the publication. A screenshot of the publication's graph was taken and pasted into the spreadsheet. Given that the graph from the spreadsheet (in this case, Numbers for Mac) is transparent, one can easily overlay the graph onto the publication's image and adjust the size to align the axes. If the two graphs match with pixel precision, then the discrepancies between the pharmacokinetic datasets and simulations are minimal.
The primary aim of composing this blog post was to showcase the user-friendly nature of Tivatrainer in conducting such intricate simulations. However, the analysis of Absalom et al.'s publication consumed the available space for the blog. Consequently, the introduction of the Eleveld propofol model in lieu of the Peadfusor model will have to be postponed to the subsequent blog entry. Nevertheless, I can disclose that the outcomes did indeed catch me by surprise.