Remimazolam Pk for children

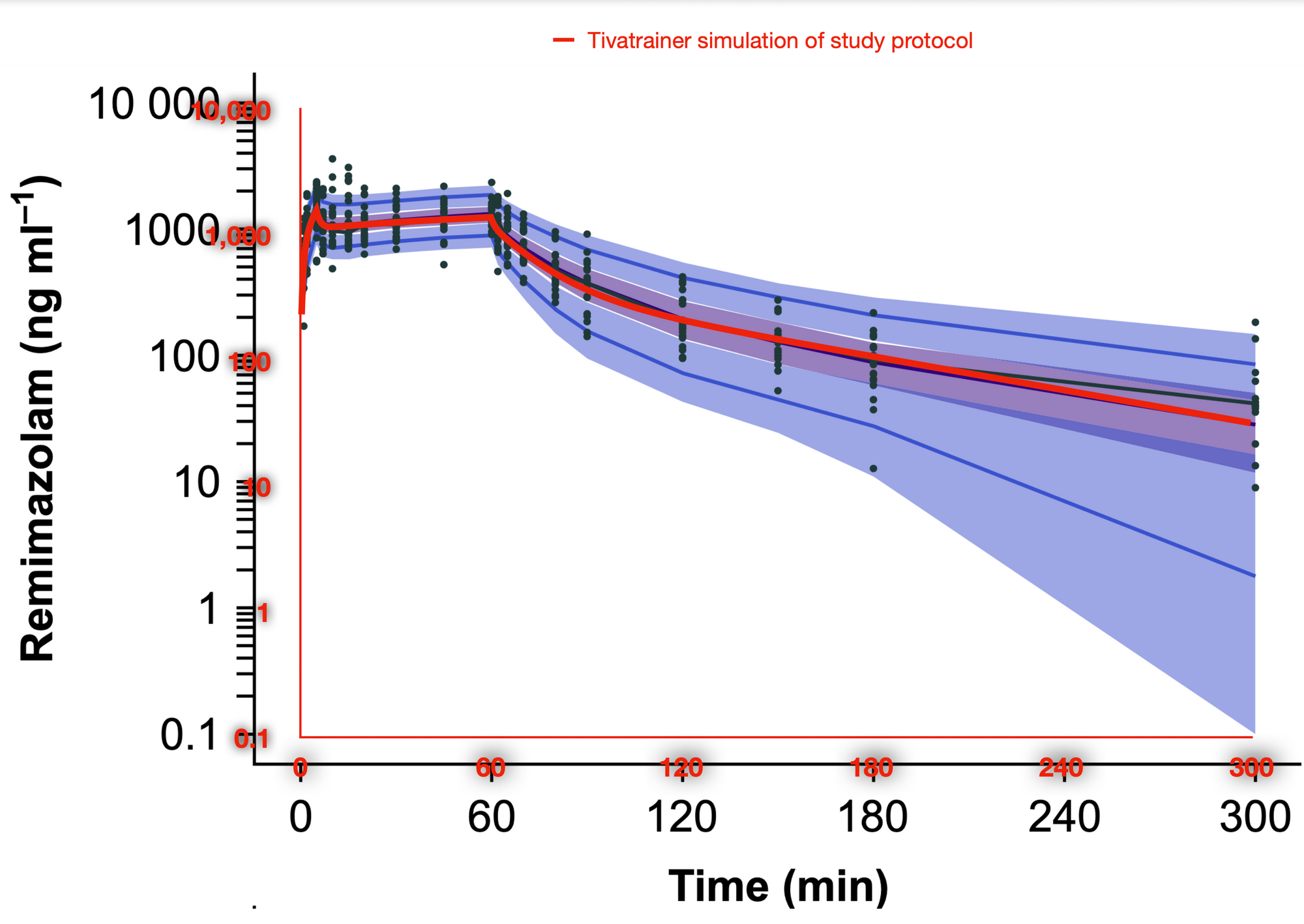
From analysis of the use of the latest version of Tivatrainer we know that many simulations are done with the relative new sedative Remimazolam. So when a preprint of the British Journal of Anaesthesia published a study on the pharmacokinetics for children, I implemented that in Tivatrainer. Tivatrainer by the way, now is growing more and more mature with saving files and export possibilities, but that will be the subject of another blog. The Pk study was performed in a limited number(23) of children aged 3-6 yr. This limited number reflects the complexity of doing these studies in children. The parameterisation of the model is not very complex: the clearances: Cel, Q2 and Q3 are scaled allometrically to a weight of 70 kg with a 0.75 power ((weight/70)^0.75) while the volumes are scaled to 70kg with the power of 1(that is weight/70 for non mathematicians). Not age, gender or another coparameter influences the model, which is understandable considering the similarity of the anthropometrics in the study population. After implementing these parameters in Tivatrainer, a simulation was made using the dosing scheme of the study in a patient with a weight of 18 kg: the mean weight in the population. Using the new export function of Tivatrainer (total simulation time 1800 sec with 20 sec interval) and importing the data in Mac numbers, a graph was made of the central concentration using a logarithmic scale on the concentration axis. It is important to mention that the interval only limits the number of graphical points not the precision of the simulation as the calculations in Tivatrainer are done analytical and not numerical. If numerical methods are used like in a spreadsheet then the exponential curves would be considered to be an addition of horizontal lines of a duration equal to the interval. A visual check with the published data confirmed that the implementation of this Pk set in Tivatraine is correct.
The authors suggest that the difference between the adult Pk and children is not that extreem. Tivatrainer is very useful for comparing different models, different patients, different modes of administration and more. I made two simulations in Tivatrainer and exported the concentration data to a spreadsheet for graphing. The first one is the child Pk and infusion protocol from the above publication compared with the adult model from Masui. Infusion was conform the study 5 mg/kg for 5 minutes and then 1.5 mg/kg for 55 minutes.
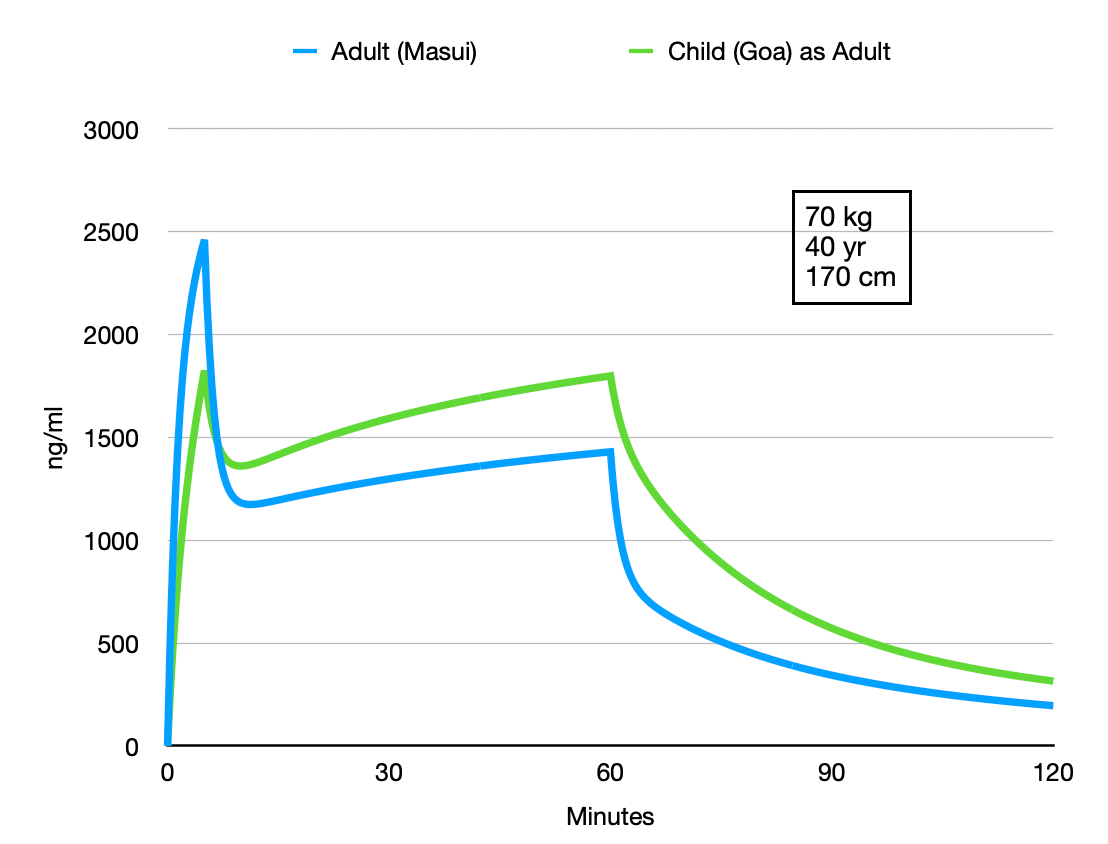
This comparison shows that it maybe not a good idea to use the childrens Pk for adults(if you ever considered that). Because of the difference in the size of the central compartment adult:3.57 L child as adult:7.56 L the peak concentration in the adult is higher: less volume to dilute the same dose of 5 mg/kg/hr. During the next infusion of 1.5 mg/kg/hr that is supposed to create a 'maintenance' the 'child as adult' central concentration rises more that the adult Pk. Finally the 'child as adult' as has a lower clearance and starts at a higher concentration point, predicting considerable higher concentrations.
What if we swap this simulation: using the adult Pk from Masui to simulate the average child in the publication of Gao et all.
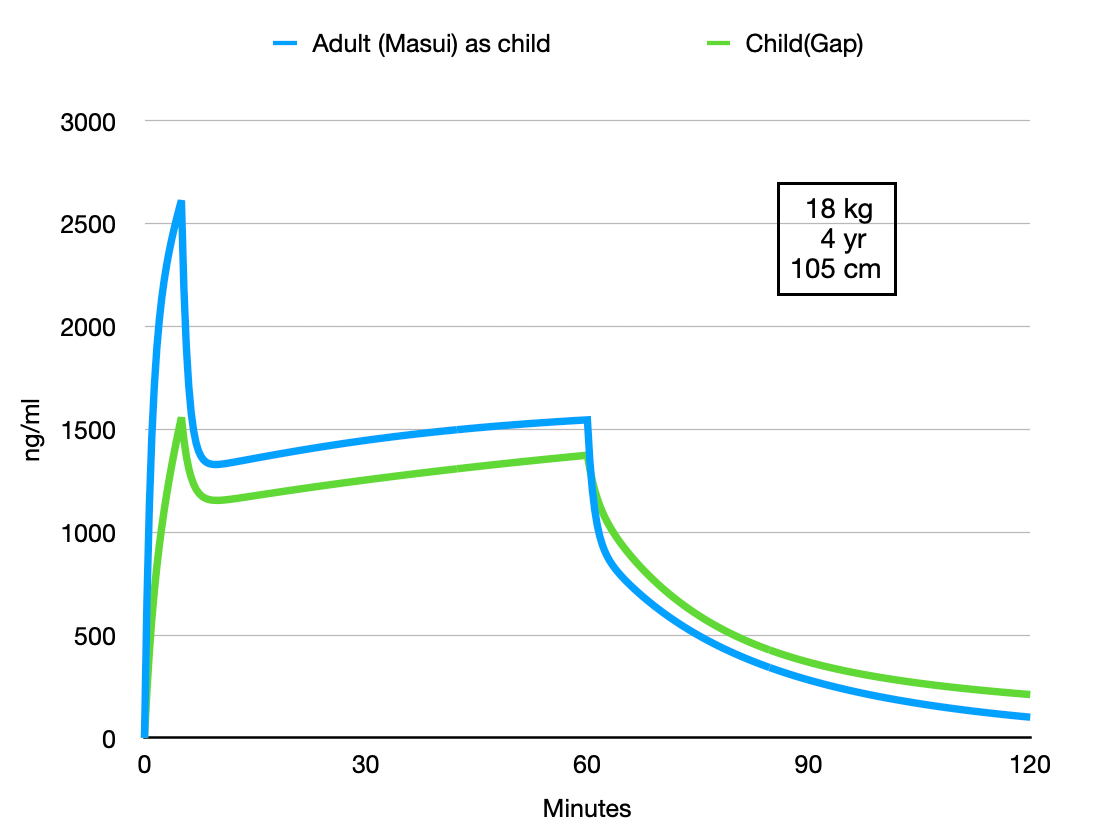
When using the adult Pk with the patient parameters of the Pk study for children the differences are less extensive appart from the initial peak concentration that is still the consequence of the different size of the central volume. The rest of the curves are more parallel and if it were not for this initial concentration difference I doubt you would notice the difference in clinical practice. The authors suggest that their study data could be used for TCI with remimazolam for children. Although these simulations could be extended to analyse differences when using these models for TCI and ETCI, I think a bit more data is required before a general purpose model like the Eleveld model for propofol will become available for remimazolam.