From Schnider to Eleveld
From Schnider to Eleveld.
This blog is about what to expect if you change the pharmacokinetic model in your TCI device from Schnider to Eleveld and how to prepare for it.
If you are using Target Controlled Infusion (TCI) for Propofol you are most likely, and hopefully, aware that there is more than one pharmacokinetic model that can be implemented and used in the TCI infusion device. Let’s refresh:
Before the new global population Eleveld model became available, there were two Marsh models and two Schnider models. Why two of each? Each model has two different implementations of the blood-brain equilibration constant, keo. This keo plays an important role when the TCI system is used in effect site control mode. The origin of the differences between these two implementations of the Schnider model can be found in the literature[1]. Here are some key points concerning the Schnider model.
The pharmacodynamics in the Schnider[2] model is based on the analysis of the canonical univariate parameter of the processed EEG (which is not the BIS!!). This could be the reason the keo in this analysis is larger (Thalfkeo is faster) than all other estimated keo values for the Schnider model. In the original publication, the keo is the same for all subjects. For the other implementation in commercial TCI systems, the keo is based on the Time to Peak Effect (TPE)[3]. If the TPE is the same for every patient but the kinetics are different for each patient then the keo must be different to produce the same TPE.(see also the blog about Sufentanil) The reason why this specifically affects the Schnider model lies in the complex clearance calculation: 0.0456 x weight+ 0.0264 x height - 0.0681 x LeanBodyMass -2.2761. So every combination of weight and height will produce a different clearance. This makes the TPE(that is dose independent) dependent on anthropometrics. For models that are linearly related to weight like the Marsh model the TPE will be the same for all patients. For more complex models (also the Eleveld model) the TPE will be different for each patient. The fixed TPE implementation for the Schnider model was selected by some open TCI systems because it produced a larger induction dose, particularly in obese patients that was considered more appropriate.
Both implementations bear the same name, which can be very confusing, as a colleague recently discovered[4]. Which one is used depends on the brand of the TCI device.
Furthermore, due to an error in the Lean Body Mass (LBM) calculation, the upper weight limit for the male population is set to a Body Mass Index(BMI) of 42 kg/m2 and 37 kg/m2 for females. Above these limits, the LBM, which is a co-parameter of the central clearance, decreases and the clearance increases. The central compartment is small and fixed to 4.27 L. So, in plasma-controlled mode, every subject will get the same small induction dose for the same target (target x 4.27 mg). Therefore, effect site mode is recommended[5] to obtain an adequate induction dose, with the induction target recommended to be about 3 times the maintenance target.
If you are using the Schnider model, you are accepting that you are using a model with a proven error in the LBM calculation, a too-small keo, input parameters that are limited at a certain not easy to understand way and two different implementations existing in the clinical environment with the same name.
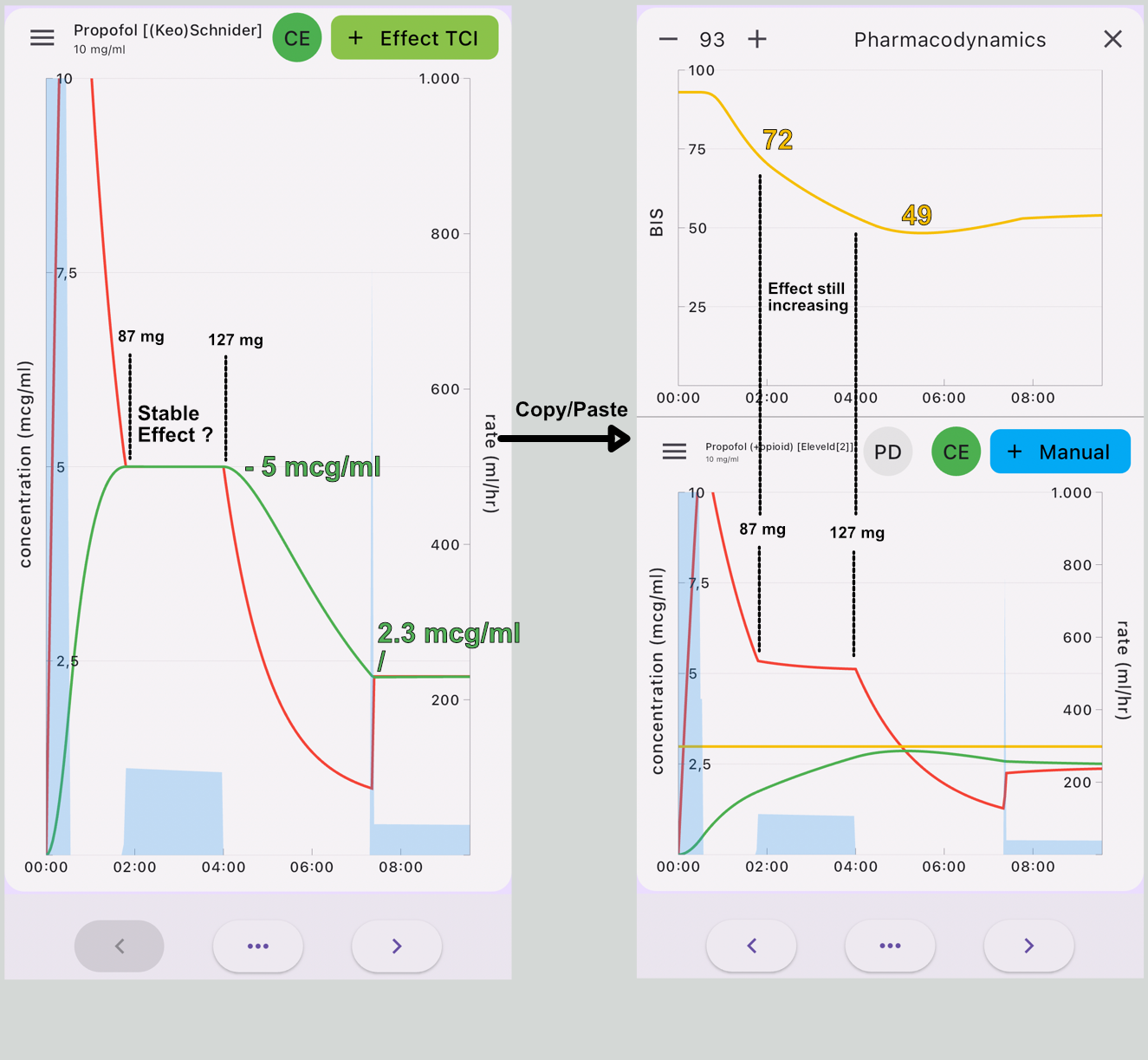
So, for good reasons, you might want to switch to the Eleveld model[6]. Getting the induction dose right is an important goal in IV anaesthesia. In a recent publication, Thomas Schnider revealed that in a post hoc analysis of the clinical application of the Schnider model in 4584 cases, the median Effect Site concentration at 30 minutes (Cet30) was 2.3 mcg/ml using the Schnider model[7]. In another clinical study comparing Schnider with Eleveld, the maintenance concentration was 2.4-2.3 mcg/ml[8]. So, targeting the effect site concentration to 2.3 mcg/ml should be sufficient for induction, right? Unfortunately not. Using effect site control in the Schnider model, an effect target of 2.3 mcg/ml would be achieved in 1.5 minutes by delivering only 40 mg of propofol, which is clearly not an adequate induction dose. So, guided by experience and expert advice, a higher induction dose is usually selected[5]. Assuming you used effect site control Schnider with an initial target of 5 mcg/ml, at obtaining the target with a dose of 1.1 mg/kg, a considerable number of your patients will not have lost consciousness, and although the effect site predicts a stable effect, most patients will achieve loss of consciousness (LOC) while maintaining this target. If after induction, the target is reduced to 2.3 mcg/kg. The dose at 4 minutes now is 1.8 mg/kg.
If these numbers do not match your clinical practice because you use ‘the other’ Schnider model or use more than one step, you can use Tivatrainer to simulate your standard induction with the Schnider model. Both (Keo)Schnider and (TPE)Schnider are available in Tivatrainer. Ask the pump manufacturer which model is implemented. (Hope they know!)
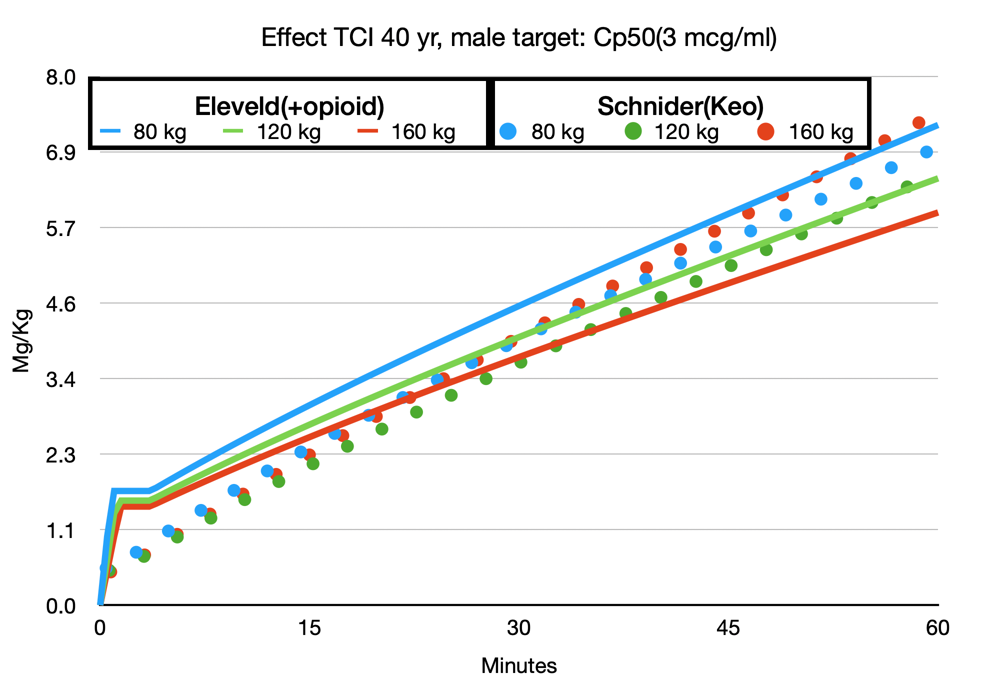
The following simulation(fig 1) is copy-paste from Schnider Effect site control to Eleveld Manual mode, so the Eleveld model calculates the concentration based on the dose given in the Schnider simulation. In maintenance, the concentrations are almost perfectly parallel for the standard patient (70 kg, 170 cm, 40 yr, male). The 2.3 mcg/ml in Schnider corresponds with 2.4 mcg/ml in the Eleveld model. The effect site in the Eleveld at the point of obtaining 1.8 mg/kg is almost exactly the Ce50 in Eleveld, which corresponds with 3 mcg ml. However, when the effect site concentration is predicted to be stable at 5 mcg/ml with the Schnider model, the Eleveld model predicts a still increasing effect, which will be consistent with your observations that, while maintaining a constant effect site concentration in the Schnider model in reality the effect increases. So, when using the Eleveld model you can aim for the Cp50Bis and then decrease to a maintenance target of 2.4 mcg/ml or better yet, target 2.4 mcg/ml immediately and titrate up or down if necessary.
Avoid selecting higher induction targets like you used to do with the Schnider model.
Maintenance targets in the Eleveld model are slightly higher than in the Schnider model (about 5%)but only in an average sized patient.
Together with Dr. Hernán Boveri, we will discuss some induction strategies followed by multimodal anaesthesia in an upcoming publication that will be announced in this blog.
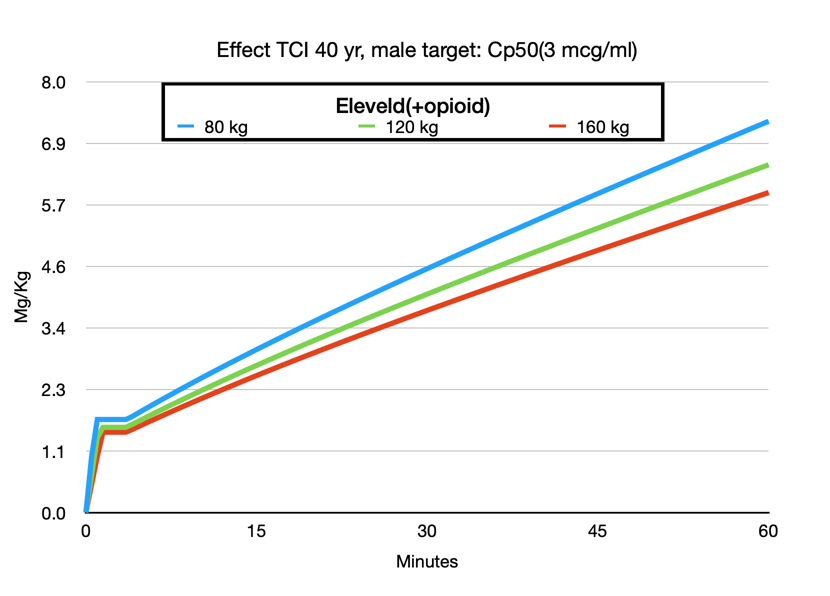
One of the most attractive features of the Eleveld model is that it can be used across a wide range of patient groups: children, obese, and elderly patients. No need to switch to Paedfusor or consider total body weight, ideal body weight, or use the Servin formula, etc. Of course, all models are wrong, but some are more wrong than others, or if you prefer a more positive phrasing: some are useful. Much has been published about the use of TCI in the obese patient. The adaptation of the Eleveld model to weight makes sense. Increasing weight increases the dose but not in a linear fashion. It is not difficult to understand that a 140 kg patient is not equivalent to two 70 kg patients. This is exactly what the Marsh model assumes with the linear relationship between weight and concentration. The Schnider model behaves even more irrationally in extremely obese patients when not limited to the maximum BMI because of its erroneous Lean Body Mass calculation.
Ce50
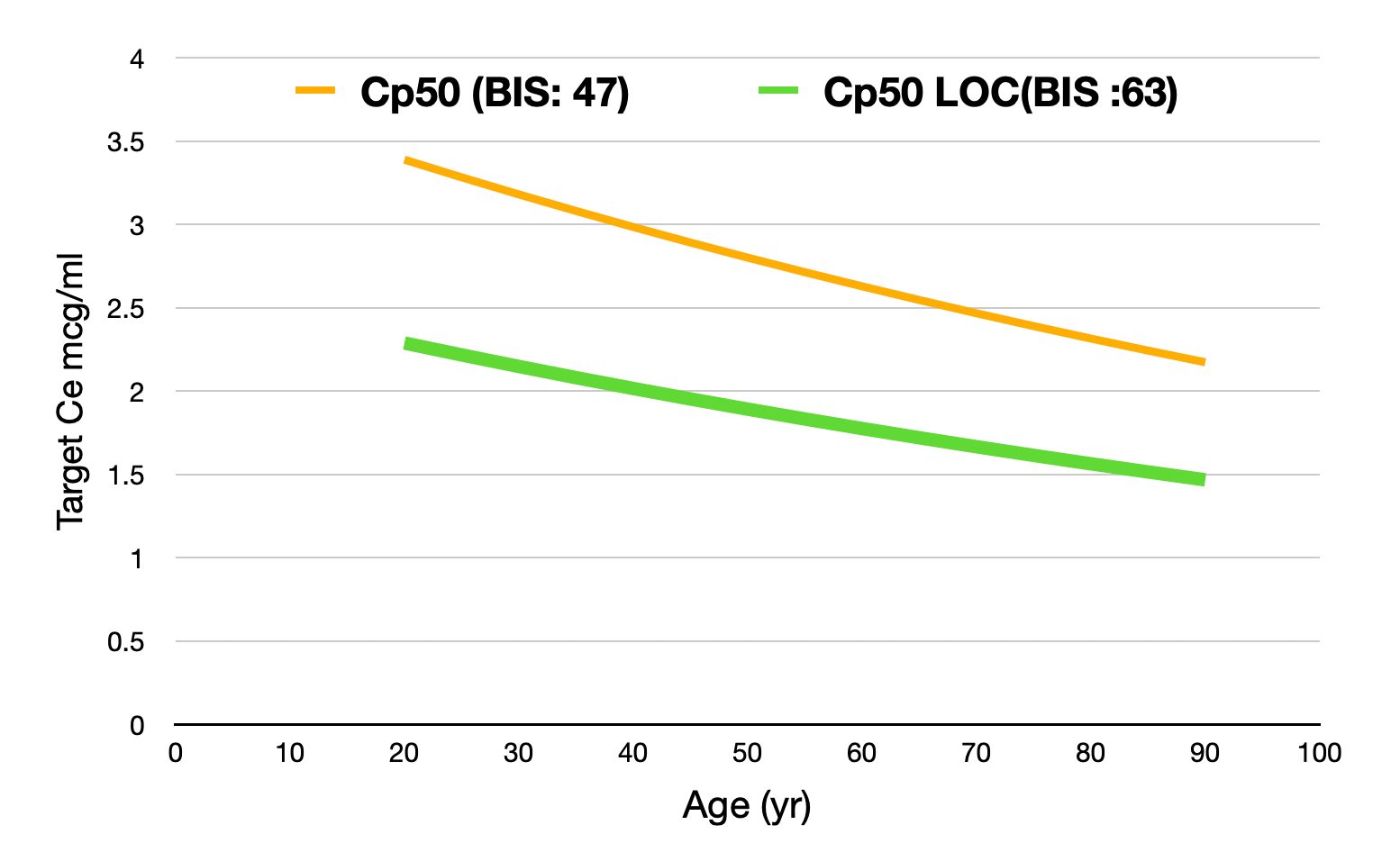
In Tivatrainer, you will see a yellow line in the Eleveld simulations. This line corresponds with the Ce50 of Propofol. In the Eleveld model, Ce50 is related to a BIS value being the midpoint between the BIS awake (93) and the lowest possible BIS: 0. Therefore, Ce50 corresponds to a BIS of 47.
For Loss of Consciousness (LOC) in a population, a traditional Ce50Loc corresponds with 50% of the patients having LOC and 50% being awake.
The Ce50 in the Eleveld model is NOT equivalent to 50% of the patients having LOC.
Depending on the method used for determining LOC, the associated BIS at the point of LOC is around 60-65.
If LOC is what you are aiming for, then targeting Ce50 BIS in Eleveld will result in a too high effect site concentration.
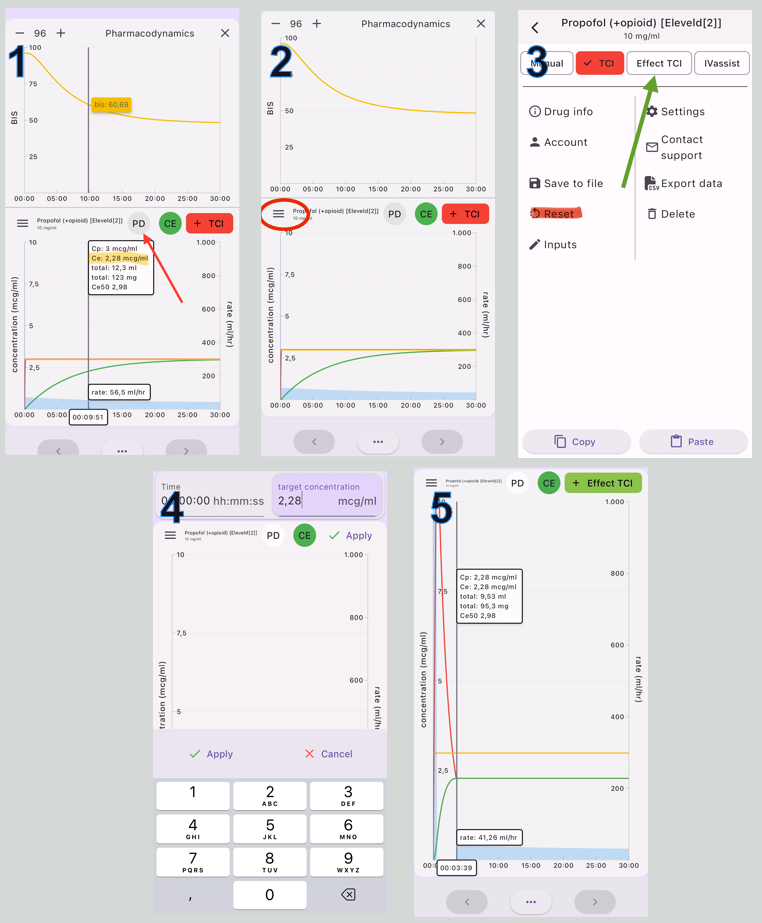
You can use Tivatrainer to set your target to a 50% probability of LOC assuming this corresponds to a BIS value of about 60-65. Follow the steps in figure 3.
Substituting the BIS value for the concentration in the effect compartment required to obtain a BIS target of 63 shows a reduction of the Cp50 with about 30% related to age.
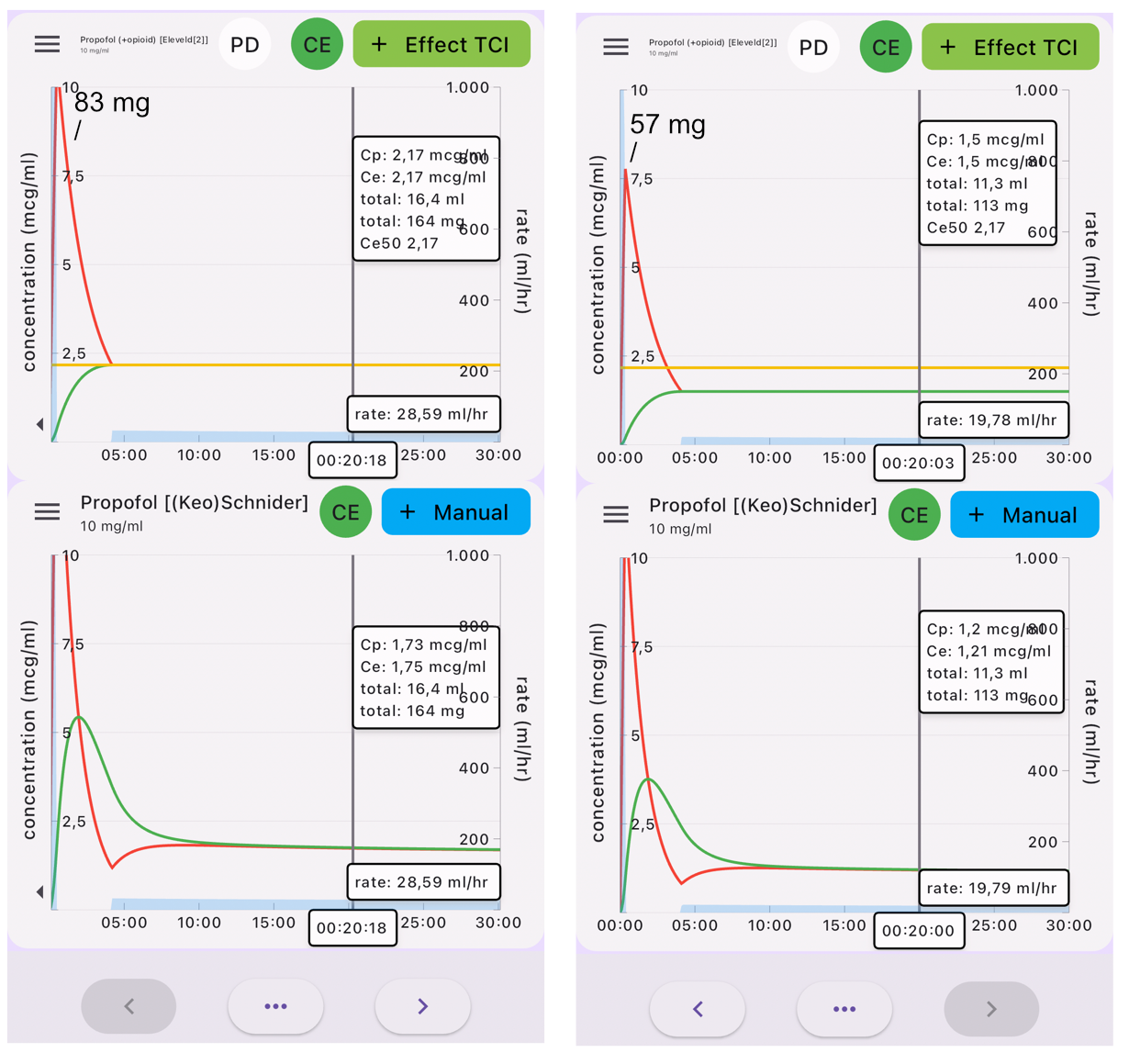
From Eleveld to Schnider
Turning things around can give you an impression of how the Eleveld model compares to the Schnider model you are used to. The implementation of the Eleveld model may not be generally available yet, but you can use the Tivatrainer to get an impression of how the Eleveld model will behave in less standard patients.
Finally, and this has been said before: we can keep disputing about the performance of specific model in a specific situation with dodgy arguments and statistics[9] but there is no doubt in my mind that the advantages of having one model in a TCI system that is universally usable and respects the dosages that have been defined in the Summary of product characteristics (SPC) is the future of TCI and TIVA. [10]
references:
-
Engbers, F. H. M. & Dahan, A. Anomalies in target-controlled infusion: an analysis after 20 years of clinical use. Anaesthesia 73, 619–630 (2018).
-
Schnider, T. W. et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology 88, 1170–1182 (1998).
-
Minto, C. F., Schnider, T. W., Gregg, K. M., Henthorn, T. K. & Shafer, S. L. Using the Time of Maximum Effect Site Concentration to Combine Pharmacokinetics and Pharmacodynamics. Anesthesiology 99, 324–333 (2003).
-
Soehle, M., Wolter, A., Thudium, M., Frede, S. & Coburn, M. Different behaviour of target-controlled infusion pumps despite apparently using the same Schnider pharmacokinetic model: An observational in vitro study. Eur. J. Anaesthesiol. Intensiv. Care 1, e011-1–8 (2022).
-
Absalom, A. R., Mani, V., Smet, T. D. & Struys, M. M. R. F. Pharmacokinetic models for propofol--defining and illuminating the devil in the detail. Br J Anaesth 103, 26–37 (2009).
-Engbers, F. H., Sutcliffe, N., Kenny, G. & Schraag, S. Pharmacokinetic models for propofol: defining and illuminating the devil in the detail. Br J Anaesth 104, 261-2-author reply 262-4 (2010). -
Eleveld, D. J., Colin, P., Absalom, A. R., Anaesthesia, M. S. B. J. of & 2018. Pharmacokinetic–pharmacodynamic model for propofol for broad application in anaesthesia and sedation. Elsevier 120, 942–959 (2018).
-
Schnider, T. W., Minto, C. F., Egan, T. D. & Filipovic, M. Relationship Between Propofol Target Concentrations, Bispectral Index, and Patient Covariates During Anesthesia. Anesthesia Analg. 132, 735–742 (2020).
-
Linassi, F. et al. Schnider and Eleveld Models for Propofol Target-Controlled Infusion Anesthesia: A Clinical Comparison. Life 13, 2065 (2023).
-
Schnider, T. W., Minto, C. F., Egan, T. D. & Filipovic, M. Clinical validation of pharmacokinetic and pharmacodynamic models for propofol infusion. Comment on Br J Anaesth 2021; 126: 386–94. Brit J Anaesth 126, e172–e174 (2021).
-
Vellinga, R., Eleveld, D. J., Struys, M. M. R. F. & Berg, J. P. van den. General purpose models for intravenous anesthetics, the next generation for target-controlled infusion and total intravenous anesthesia? Curr. Opin. Anaesthesiol. 36, 602–607 (2023).