The keo of Sufentanil: yet another anomaly in commercial Target Controlled Infusion systems
-the keo implementation of the Gepts model for sufentanil differs between the brands of pumps due to a mis-interpretation -input weight range in one pump is 1-250 kg but the Gepts model has a fixed weight so all patients get the same amount of Sufentanil
In 2018, I authored a publication addressing the "anomalies" observed in commercially available target controlled infusion (TCI) systems. In order to maintain the appropriate tone for a scientific journal, I refrained from using terms such as "mistake" or "stupid." However, it is fortunate that there is greater flexibility in expressing ideas within a blog format.
The Gepts model for Sufentanil
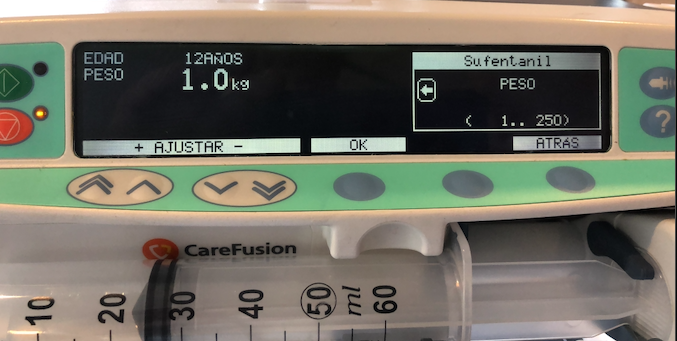
In my professional opinion, the implementation of a pharmacokinetic model that lacks any consideration for patient characteristics, not even the weight, is simply imprudent. It can be considered ignorant when utilized without strict patient selection criteria. To our surprise, this particular model demonstrated satisfactory performance in (E)TCI when used on obese patients. However, the Gepts study employed a completely different anesthesia technique. The applicability of extrapolating these findings to patients weighing up to 250 kg, as depicted in the image, remains uncertain. For TCI applications, the Gepts model for Sufentanil assumes a fixed weight of 70 kg for every patient, regardless of their actual weight. However, even more concerning is the decision to set the lowest weight limit at 1 kg in TCI systems from Alaris/Carefusion, Medcaptain, Mindray and possibly others. It is possible that during the development process, the existence of a pharmacokinetic model for neonates was acknowledged but never implemented. Although the minimum age requirement is 12 years, a colleague who disregards correctly age as a parameter that is not part of the Pk model and vaguely recalls Sufentanil Pk studies in neonates, might be tempted to use the TCI system in very, very young patients, leading to disastrous outcomes.
If even the slightest hazard affecting children were to arise in the car industry, it would necessitate a complete recall of the implicated vehicles. However, when it comes to TCI systems, no one appears to assume responsibility. In Europe, the European Medical Association (EMA) regards target controlled infusion pumps as devices that require certification from regulatory bodies responsible for setting standards related to accuracy and safety. However, the standards do not encompass the selection and appropriate application of a pharmacokinetic model. In contrast, the FDA in the United States has not approved target controlled infusion pumps.
Is the responsibility for the Pk/Pd model and its usage placed on the users?
However, this is not the sole issue with Sufentanil TCI. Sufentanil has been integrated into the Tivatrainer for quite some time. It was only recently brought to my attention that the implementation of the Thalfkeo for sufentanil in Tivatrainer did not align with the implementation in the commercially available TCI systems. Therefore, I embarked on a search through publications to identify the source of this disparity.
In one of the early studies examining the impact of opioids on the EEG, a Thalfkeo (T1/2keo) of 6.2 minutes was determined for sufentanil. This was achieved by constructing a hysteresis loop between the measured blood concentration, which increased through fixed rate infusion, and the continuously monitored effect. Since the pharmacokinetic data could not be utilized to construct a pharmacokinetic model, the identified Thalfkeo was non-parametric, meaning it did not belong to a specific pharmacokinetic model. It is important to note that a given drug dose can result in different blood concentrations depending on the used pharmacokinetic model. Connecting these theoretical blood concentrations to the effect necessitates different equilibration times. By substituting the blood concentrations in the hysteresis loop with calculated concentrations from the pharmacokinetic model, collapsing the loop will yield a parametric Thalfkeo, which corresponds to the specific pharmacokinetic model. Various methods have been proposed to determine the Thalfkeo that corresponds to a pharmacokinetic model, with one popular approach being the measurement of the time to peak effect (TPE) after a bolus and determining the Thalfkeo that aligns with this TPE. However, it is important to acknowledge the limitations of this method, which will be discussed in another blog.
As an example of the Time to Peak Effect method I have included a video where the TPE of the Eleveld model is calculated and a mutable Keo is implemented. This not only demonstrates the technique but also the uselessness of the TPE in target controlled infusion.
Comparison between the original Keo in the Eleveld model derived from the sigmoid Emax model and the TPE method. The observed TPE for propofol is 1.6 minutes. This demonstrates the uselessness of TPE for Target Controlled infusion.
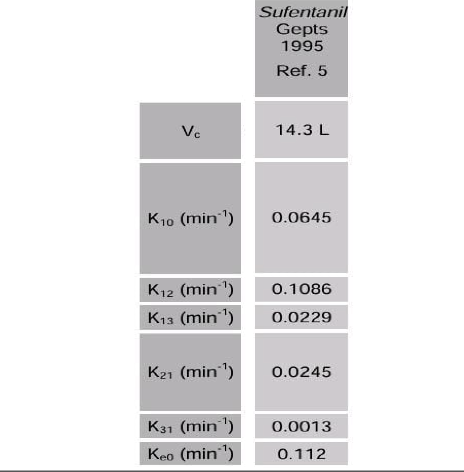
In summary, the concept is straightforward: administer an appropriate bolus dose, monitor the effect continuously, identify the peak, and adjust the Thalfkeo so that the peak effect corresponds to the predicted peak effect of the model. In another publication, the non-parametric Thalfkeo from the study above, was utilized for comparisons between opioids. Since the original study did not provide any published pharmacokinetic parameters to determine the Thalfkeo, a different pharmacokinetic set was employed for the comparison simulations between Sufentanil and the other opioids. One of the simulations involved the time to peak effect using this pharmacokinetic set. When the Gepts parameters were later implemented in the TCI system, this simulated TPE was mistakenly used to establish a connection between a Thalfkeo and the Gepts model.
Instead of utilizing a measured Time To Peak Effect, certain pump manufacturers such as Alaris/Carefusion, Medcaptain Arcomed (and potentially others) employed a simulated TPE of 5.6 minutes in order to determine a Thalfkeo of 3.05 minutes (keo: 0.17559 /min). This incontrast to other manufacturers like Fresenius with their base primea model (and potentially others) who correctly employed the non-parametric Thalfkeo of 6.2 minutes (keo: 0.112/min).
[Note: keo= ln(2)/Thalfkeo]

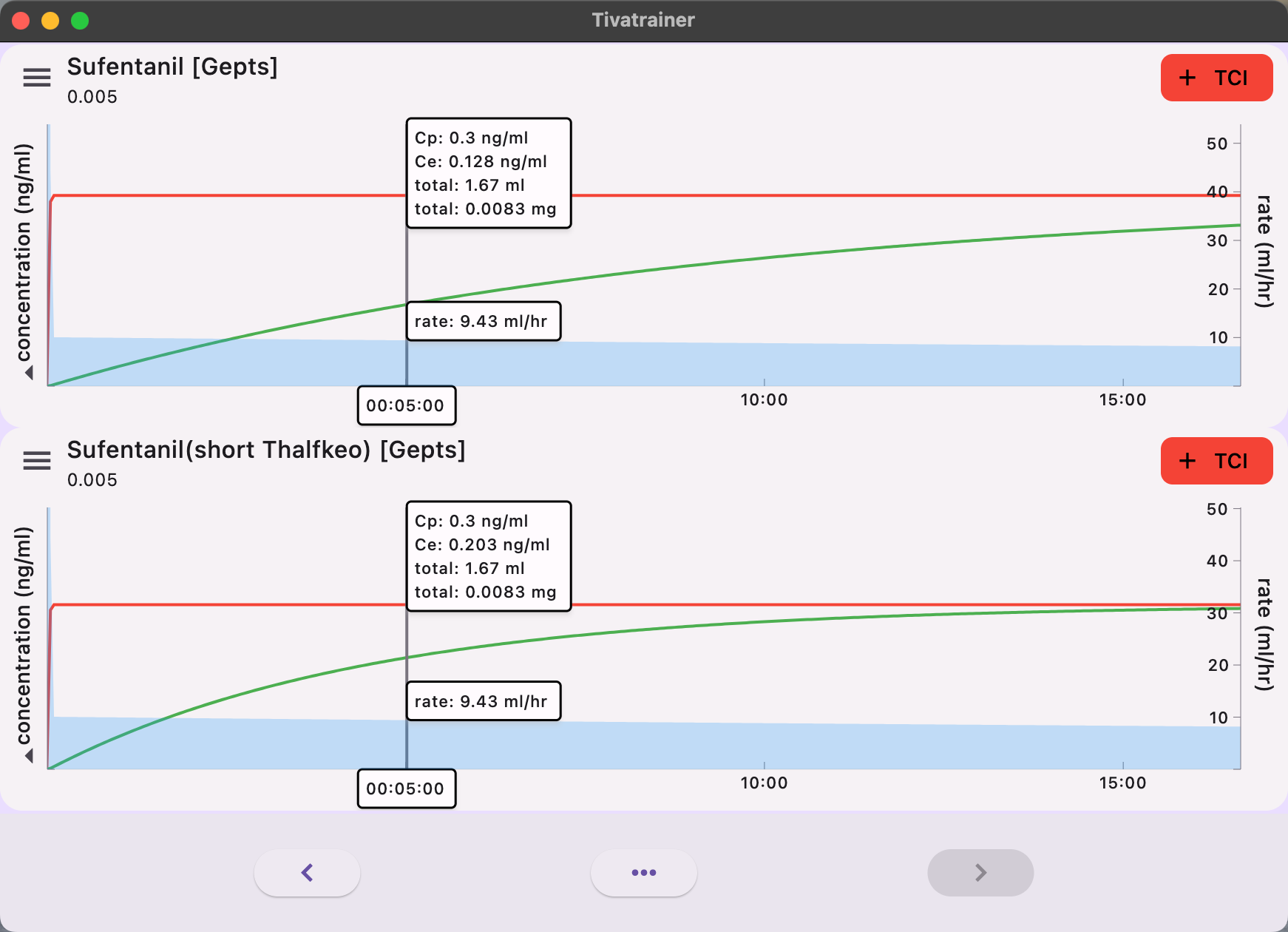
The implications of these variances are readily apparent in simulations with the Tivatrainer. In the implementation of plasma-controlled total intravenous anesthesia (TCI), the equilibrium of effect site concentration is predicted to occur more rapidly in the Alaris/Carefusion implementation compared to the Fresenius implementation. There will be no disparity in the dosage administered through plasma control. Although this may be somewhat perplexing from a clinical perspective, significant discrepancies in the induction dose will arise when the desired effect is targeted.
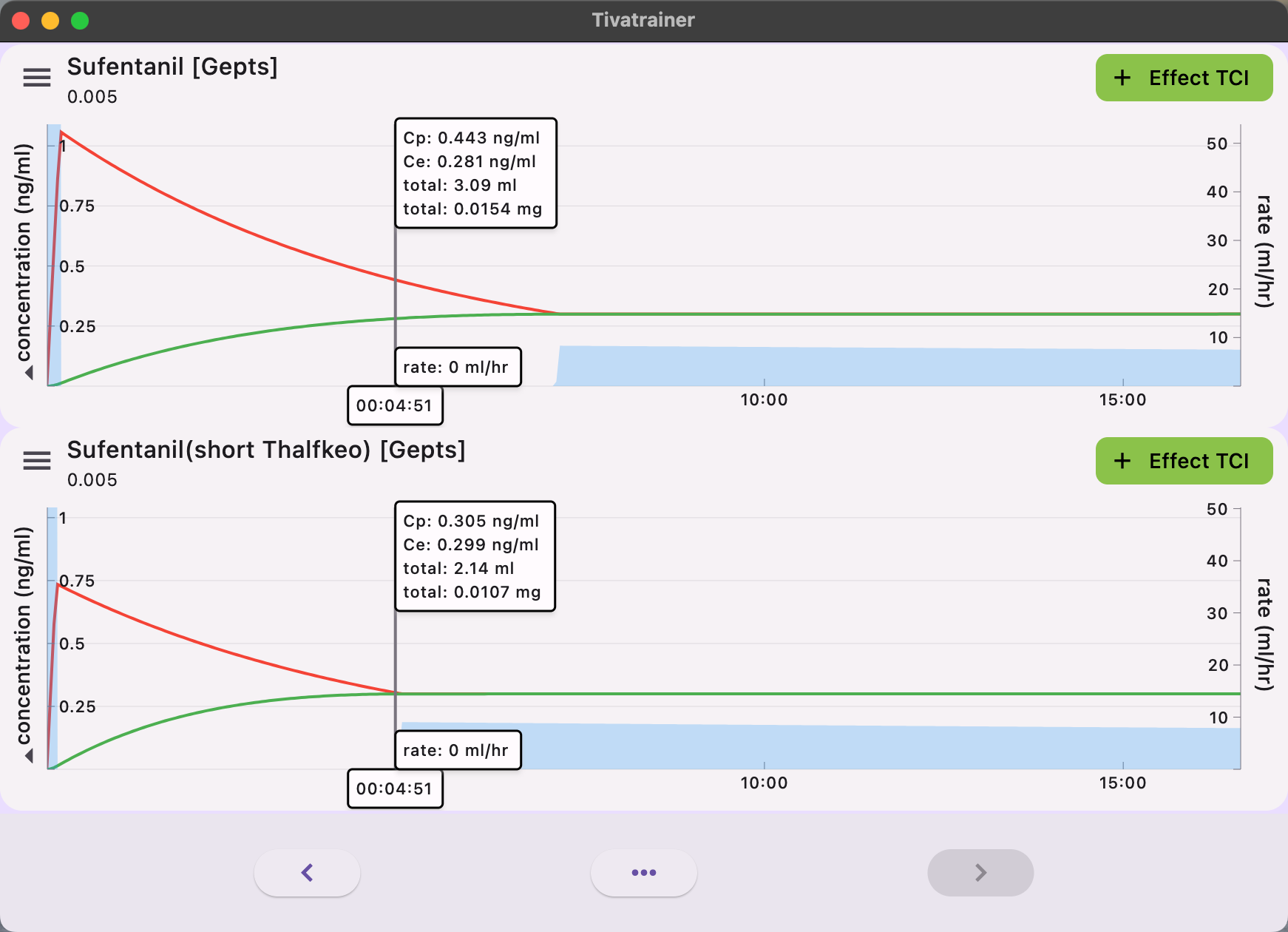
The administration of anaesthetics involves a dynamic process that requires the expertise of anaesthesiologists. Through their training and experience, they develop an intuitive understanding of the pharmacokinetics/pharmacodynamics (Pk/Pd) model, allowing them to optimize the dosing of anaesthetics. However, when using target controlled infusion (TCI) pumps, the anaesthesiologists' internal model must account for any deviations in the Pk/Pd model implemented in the pump. This becomes apparent when clinicians encounter different models for the same drug in different infusion pumps, as seen in cases where hospitals change the brand of the pump. In these situations, patients may exhibit different responses despite using the same Pk model in name: the Schnider model for propofol, but implemented differently. This issue has also been observed in a large German hospital where pumps from different brands were used. Now, the introduction of another drug, Sufentanil, with different implementations in commercially available infusion pumps for TCI adds to the complexity. Target Controlled Infusion was intended to facilitate thinking, communication, and knowledge exchange by using concentrations rather than time-dependent dosing protocols, which can be challenging to compare. However, despite the widespread use of TCI systems, it has yet to bridge the gap between scientific interest and clinical applicability and impact. To date, there is no scientific evidence that proves the superiority of one model over others, based on clinical outcome criteria. In my opinion, the way forward is to centralize and certify the models used for TCI, striving for a one drug-one model approach wherever possible. The selection of the model should consider factors such as ease of communication and training, safety by avoiding confusion, and adherence to the dosage recommendations outlined in the Summary of Product Characteristics (SPC).
It is worth noting that Eurosiva is currently working on a proposal to achieve this goal.